|
Current and recent past projects are described here. Where applicable, papers  that have been published as a result of the project are linked below the description. For older projects, please see my complete CV. that have been published as a result of the project are linked below the description. For older projects, please see my complete CV.
|
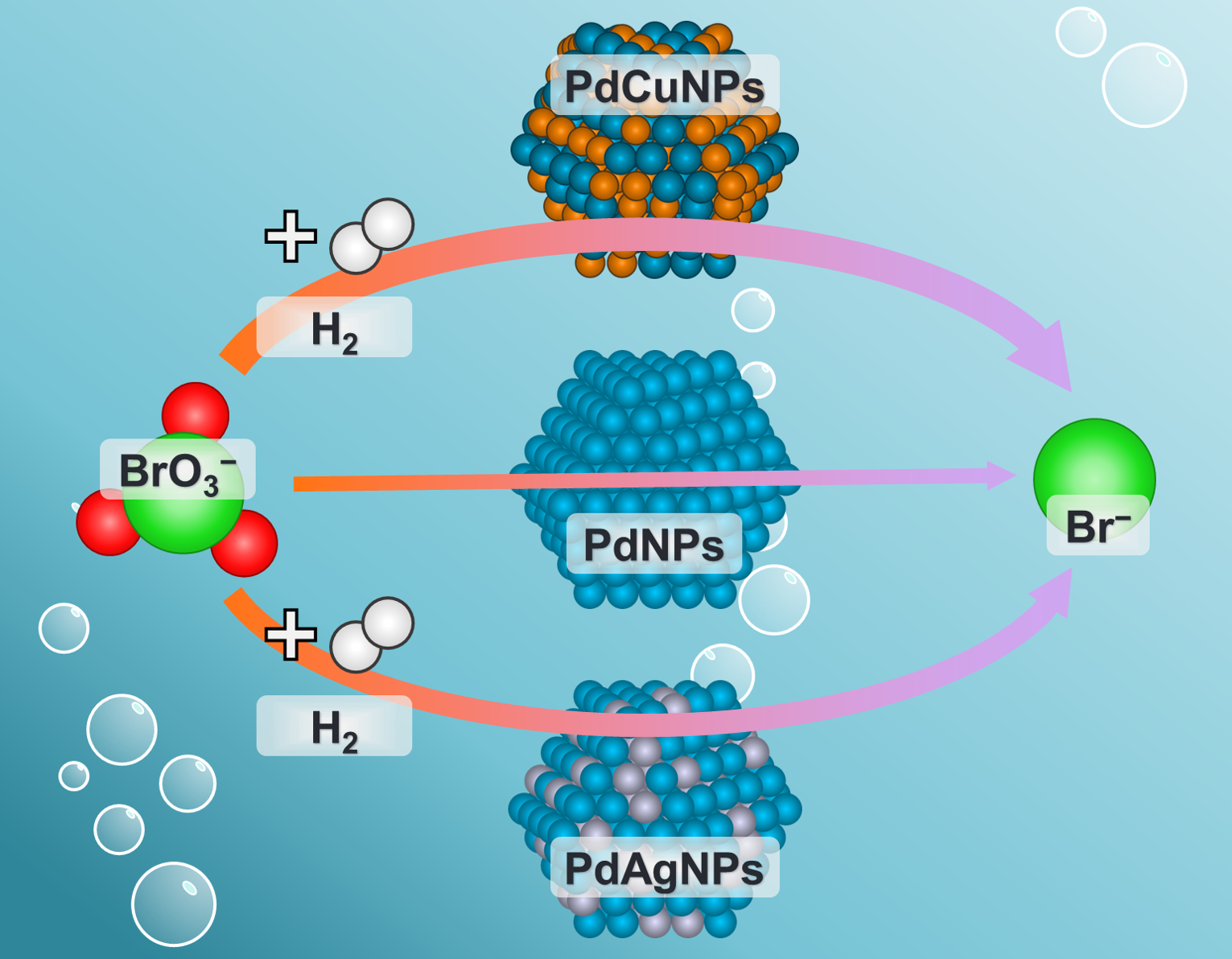 |
Enhanced Bromate Reduction via Alloyed PdCu and PdAg Nanoparticle Catalysts
• Applied Catalysis A: General, 2024
Catalytic reduction has been shown to be an effective option for the removal of bromate (BrO3−) in drinking water treatment. Because most catalysts rely on Pd, it would be advantageous to increase the efficiency of these catalysts or to reduce the amount of Pd needed in the catalyst. In this work, well-defined PdxCu100−xNPs and PdxAg100−xNPs were used to investigate the mechanism of how alloyed Pd-based materials improve BrO3− reduction activity. Experimental results combined with density functional theory calculations demonstrate that improved BrO3− reduction on PdCu catalysts is due to electronic effects generated through charge transfer between Pd and Cu atoms; on PdAg catalysts, though, improved activity is due to the creation of ideal surface ensembles that facilitate adsorption of H and BrO3−. Additionally, it was demonstrated that such effects can be realized using traditional catalyst synthesis techniques, such as incipient wetness deposition.

|
|
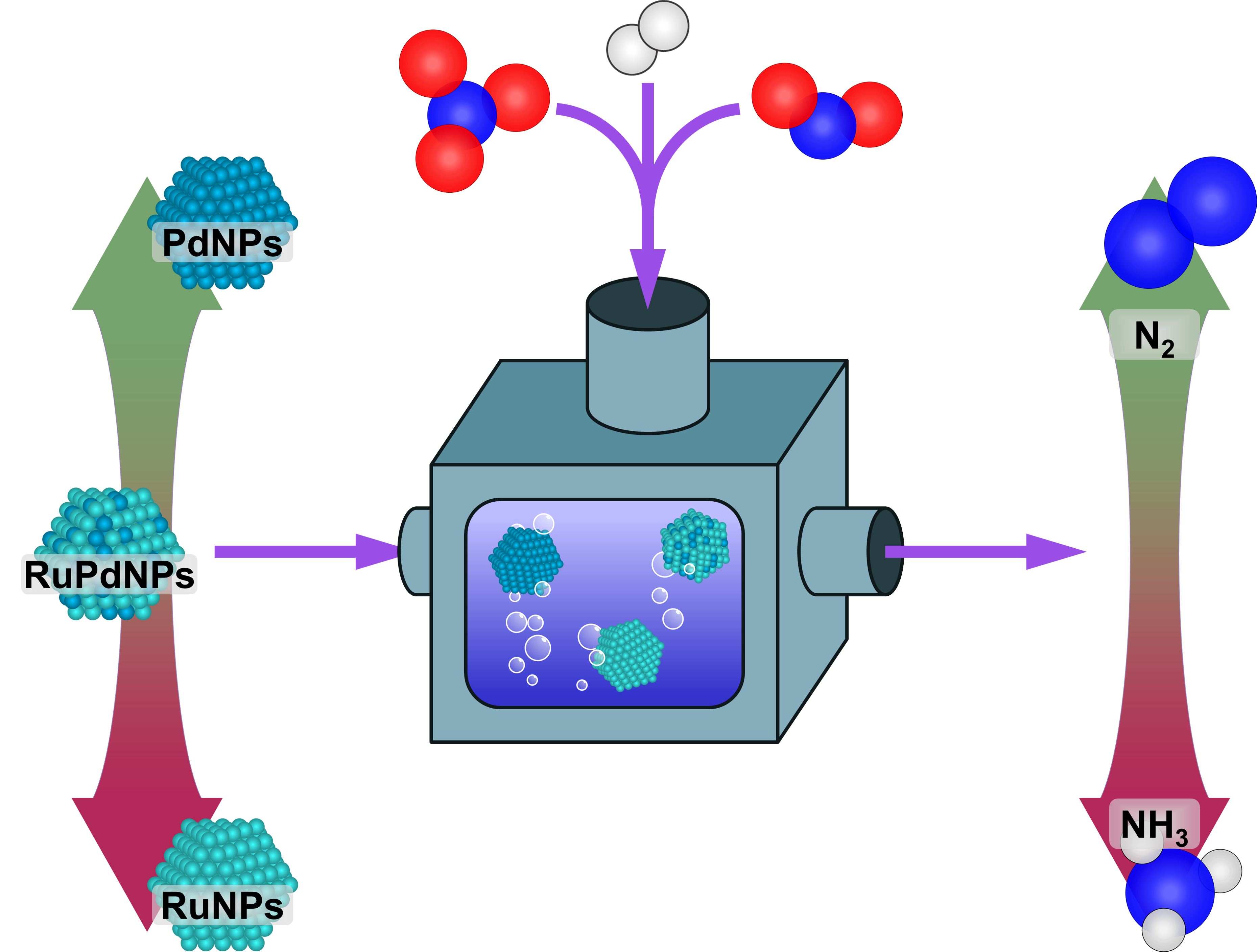 |
Tunable End-product Selectivity during NO3− Reduction via RuxPd100−xNPs
• Small, 2024
Herein, aqueous nitrate (NO3−) reduction is used to explore the composition-selectivity relationship of randomly alloyed ruthenium-palladium nanoparticle catalysts to provide insights into the factors that control selectivity during catalytic hydrogenation. NO3− reduction proceeds through nitrite (NO2−) and then nitric oxide (NO), before diverging to form N2 gas or ammonium (NH4+). Experimental results demonstrate that end-product selectivity is dependent on both starting reagent (e.g., NO3−, NO2−, or NO) and catalyst composition. These results were paired with density functional theory calculations to show that end-product is controlled through a combination of the thermodynamics of the competing reduction pathways and the binding energies of initial competing species.

|
|
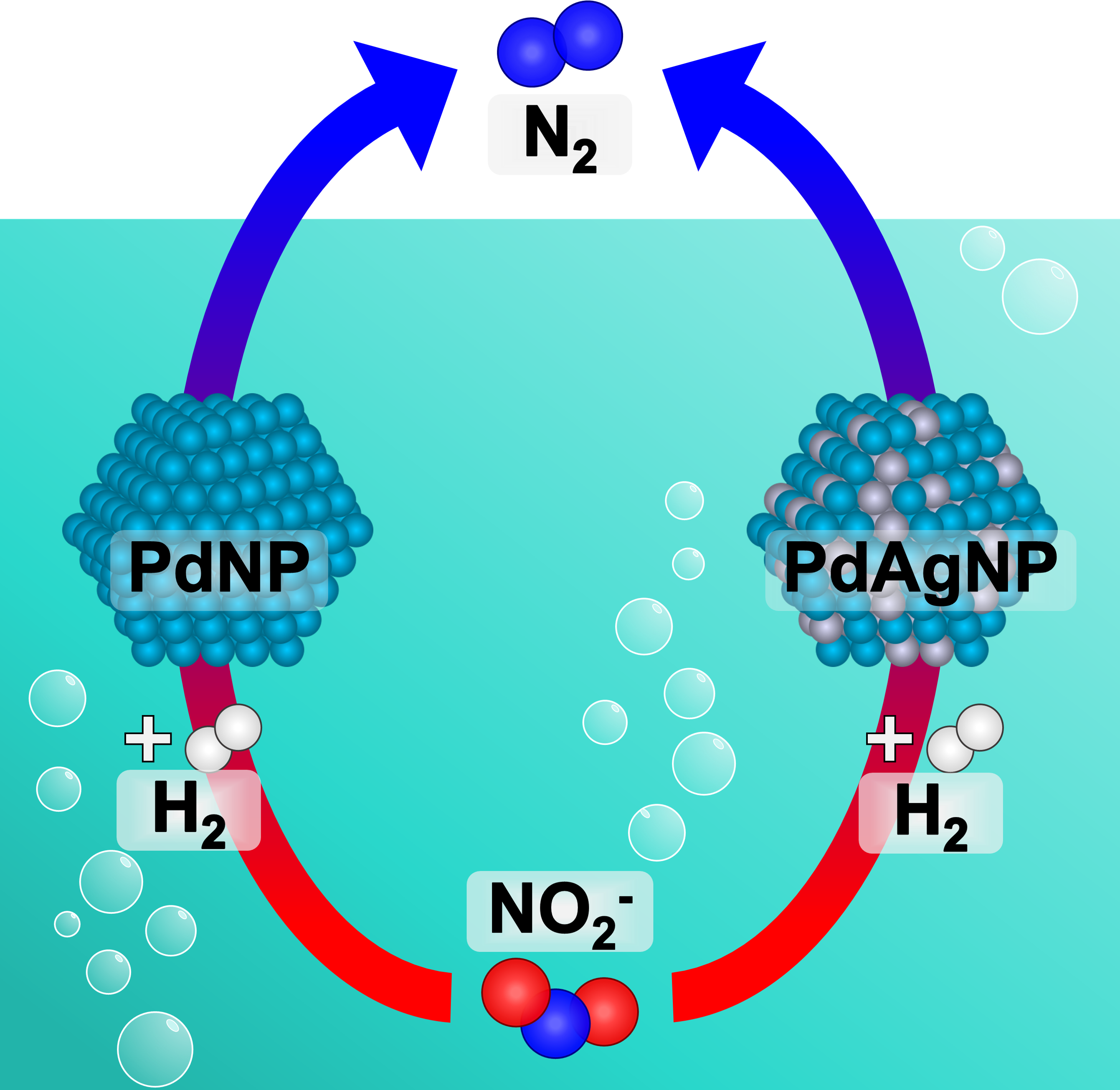 |
Alloyed PdxAg100−xNPs as Catalysts for Nitrite Removal
• ACS Catalysis, 2020
Alloys consisting of hydrogenation-active metals (e.g., Pd) and non-active metals (e.g., Au, Ag, Cu) can display enhanced reactivities due to ligand and ensemble effects between the metal atoms. Combining Pd with less expensive metals, such as Ag, also provides an attractive route for lowering catalyst cost. Herein, the effect of alloy composition (the value of x) on catalyst activity for the reduction of aqueous-phase nitrite (NO2−) was investigated. Results showed that Pd-rich particles (i.e., high values of x) showed the highest activity for NO2− conversion, though all alloys provided enhanced cost-normalized activities when compared to monometallic Pd. Density functional theory calculations revealed that ideal surface ensembles presented by alloying Pd and Ag were responsible for optimized N binding, and eventually faster reduction rates.

|
|
 |
Optimization of the Synthesis of Palladium–Silver Nanoparticles
Depsite the two metals being completely miscible across the range of composition, the synthesis of palladium–silver nanoparticles (PdAgNPs) has rarely been reported. Here, the synthesis of PdAgNPs via a microwave-assisted polyol method was optimized by testing various metal precursors, addition strategies, and heating times. Previously published strategies were adapted to prevent the loss of Ag by precipitation with counter ions. The synthesis method allowed for randomly alloyed particles with finely tunable compositions. Combining Pd with Ag in catalysts represents a convenient route for reducing the amount of Pd needed in catalysts, thus reducing financial and environmental costs, and microwave-based synthesis is an ideal method that can be easily scaled.

|



